


CHROMOSOME ABNORMALITIES IN SPONTANEOUS
ABORTIONS: APPLICATION OF MULTICOLOR
FLUORESCENT IN SITU HYBRIDIZATION AND
ORIGINAL DNA PROBES FOR CHROMOSOMES
1, 9, 13, 14, 16, 18, 21, 22, X AND Y
Vorsanova SG1,*, Kirilova EA2, Yurov YB3, Kolotii AD1,
Monakhov VV3, Iourov IY3, Beresheva AK1
*Corresponding Author: : Professor Dr. Svetlana G. Vorsanova, Director of Molecular-Cytogenetic Laboratory of Neuropsychiatric Diseases, Institute of Pediatrics and Children Surgery, Russian Ministry of Health, TaldomŽskaya str 2, 127 412 Moscow, Russia; Tel.: +7-095-484-1948; Fax: +7-095-952-8940; E-mail: y_yurov@ yahoo.com
page: 49
|
|
DISCUSSION
The main aim of this study was prospective interphase FISH diagnosis of chromosomal anomalies in spontaneous abortion specimens. We used a probe set for chromosomes 1, 9, 13, 14, 16, 18, 21, 22, X, and Y, as it well known that aneuploidies involving these chromosomes are most frequent in humans [1,2,8,9]. The results obtained allowed us to explain the cause of fetus losses in 55% of the specimens analyzed. The presence of chromosomal aneuploidies involving other chromosomes (2, 3, 4, 5, 6, 7, 8, 10, 11, 12, 15, 17, 19, 20) cannot be excluded as we have not tested for these by FISH in the present study. In recent studies, chromosome 2, 7, 10, 15, and 17 are also recognized as being involved in spontaneous abortions [3,8]. Therefore, the frequency of chromosomal abnormalities in these specimens could be higher. The set of DNA probes used is highly informative for aneuploidy screening in spontaneous abortion specimens. Recently, Jobanputra et al. [8], using interphase FISH with a probe set for chromosomes 13, 15, 16, 18, 21, 22, X and Y, have identified 54% of chromosomal abnormalities in spontaneous abortion specimens, which is similar to data obtained in the present study.
Fluorescent in situ hybridization that allows the study of aneuploidies in a large number of interphase cells could be considered as an efficient method for analysis of chromosomal mosaicism. Accurate scoring of a large number of interphase nuclei using two- or three-color FISH performed in our study, allowed us to identify mosaic forms of aneuploidies and polyploidy in 20 (51.3%) specimens with chromosomal abnormalities. Therefore, postzygotic non disjunction of chromosomes could take place in fetuses during the first trimester of pregnancy with a higher frequency than has been detected before using karyotyping techniques.
There are several reports about an excess of normal female karyotypes over normal male karyotypes in spontaneous abortion specimens [7,8]. In the present study we detected 24 normal female and four normal male karyotypes among the 63 specimens analyzed. A prevalence of female fetuses (male/female ratio 15/48) was also detected among all the specimens studied. One can propose that contamination by maternal cells could occur during specimen processing for FISH. Alternatively, the dramatic loss of male fetuses with chromosome abnormalities or lethal X-linked gene mutations, can take place in earlier stages of pregnancy thus leading to such a prevalence of female fetuses. The future direction of the study is to clarify the real cause of non random sex ratios.
The set of original DNA probes was found to be applicable for accurate and rapid screening of aneuploidies in spontaneous abortion specimens, allowing the determination of regular as well as mosaic forms of chromosome abnormalities.
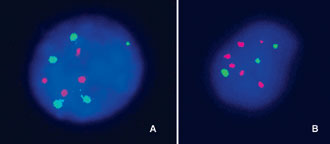
Figure 1. Examples of interphase mFISH in cells of spontaneous abortions using: A) a Cy3-labeled DNA probe for chromosomes 13/21 (four red signals) and a biotin-labeled DNA probe for chromosome 9 (five green signals) in samples with pentasomy 9; B) a Cy3-labeled DNA probe for chromosomes 13/21 (six red signals) and a biotin-labeled DNA probe for chromosome 9 (three green signals) in samples with triploidies.
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

