Linkage Analysis. Both probands and their siblings were analyzed for the following polymorphisms: R219K and R1587K of the ABCA1 gene, I/D polymorphism of ACE, M235T of AGT, eNOS4a/4b and the C/A polymorphism of EFNB3. We first determined the possibility of linkage between CAD and each of the genes studied using the S-TDT (Table 3). No gene had a z-score greater than 2.47 for CAD or for its main manifestations of acute MI and angina. Thus, none of the five genes can be considered as the main gene associated with the disease, but these genes can affect disease development. Therefore, non parametric ã correlation analysis was performed to assess the influence of the gene polymorphisms on the clinical and biochemical characteristics in the proband and sibling groups.
Table 3. inkage analysis of coronary artery disease and genes by sibling transmission/disequilibrium tests.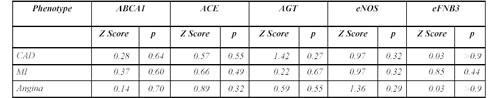
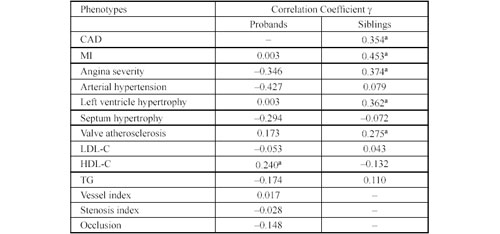
Table 4. Correlation between the ACE gene insertion/deletion polymorphism, clinical and biochemical data of coronary artery disease patients and siblings by non parametric ã correlation analysis.
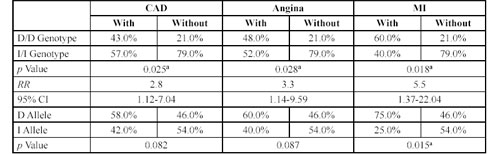
ACE Gene Insertion/Deletion Polymorphism. Table 4 shows the ã correlation between the ACE I/D polymorphism and clinical and biochemical characters in CAD patients and their siblings. In the CAD group, this polymorphism correlated significantly with the level of HDL-C. In the sibling group, there were significant correlations with CAD, acute MI, and the severity of angina, valvular atherosclerosis and left ventricular hypertrophy. Further analyses on the CAD group using the Newman-Keuls test did not reveal significant levels of correlation between the mean values of HDL-cholesterol in probands with different ACE genotypes. Table 5 shows the distribution of ACE genotypes for siblings with or without CAD, angina and MI. The DD genotype was significantly more prevalent among the siblings who had CAD than in the healthy siblings (p = 0.025). The RR for developing CAD was 2.8 for siblings with the DD genotype (95% CI 1.12-7.04). The DD genotype frequency was also higher in siblings with angina (p = 0.028), for whom the RR was 3.3 (95% CI 1.14-9.59). The most significant results were observed for siblings with MI, where the DD genotype had a RR of 5.5 (95% CI 1.37-22.04), p = 0.018.
In subjects with valvular atherosclerosis and left ventricular hypertrophy, ÷2 testing did not reveal significant associations with any ACE genotype. We conclude that the DD ACE genotype in the Russian population may be a risk factor for CAD, MI and angina, particularly for individuals having a siblings affected by CAD.
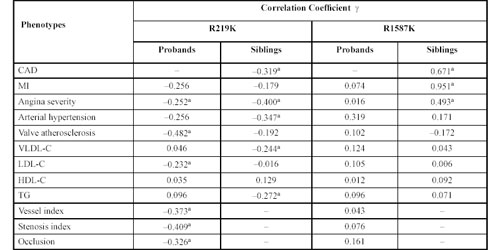
R219K and R1587K Polymorphisms of ABCA1. We tested for two common coding single-nucleotide polymor phisms of the ABCA1 gene, i.e., R219K and R1587K. Table 6 shows the ã correlations between the R219K polymorphism and clinical and biochemical data in patients and siblings. In the patient group, this polymorphism correlates with angina, LDL-C level, and especially with parameters that affect the sclerosis of coronary arteries. In the sibling group, there was a correlation with the incidence of CAD by itself, as well as with angina, hypertension and very low-density lipoprotein-cholesterol (VLDL-C) and TG levels.
Table 5. Distribution of genotypes at ACE gene insertion/deletion polymorphisms in a sibling group either with or without coronary artery disease, angina and myocardium infarction.
 Table
Table
6. Correlation between two ABCA1 gene polymorphisms, clinical and biochemical data of coronary artery disease patients and siblings by non parametric ã correlation analyses.
 For
For  Figure 1. The R219K ABCA1 gene polymorphism and mean stenosis index in CAD patients. Stenosis index for patients with KK and RK genotypes is significantly elevated compared to patients with the RR genotype (p = 0.004).
Figure 1. The R219K ABCA1 gene polymorphism and mean stenosis index in CAD patients. Stenosis index for patients with KK and RK genotypes is significantly elevated compared to patients with the RR genotype (p = 0.004).
For further analysis of correlations, we combined the RK heterozygotes and KK homozygotes into one genotype group because of the scarcity of individuals with the latter genotype. Figure 1 shows that the index of stenosis was significantly higher in subjects with the RK or KK genotypes than in those with only the RR genotype. Patients with the KK and RK genotypes more frequently showed in two or three vessel lesions (p = 0.001). Patients with the RR genotype more frequently had lesions in one vessel (Table 7).
Similar studies were carried out in the sibling group. Table 8 shows the genotype distribution in siblings with and without CAD, angina and arterial hypertension. The combination of genotype KK with RK was frequent among siblings with CAD; the RR being 2.38 (95% CI 1.02-5.80), p = 0.044. Results were similar for arterial hypertension (RR = 2.87; 95% CI 1.26-6.56), (p = 0.023) and angina (p = 0.027). The RR value for siblings with the KK and RK genotypes was 3.3 (95% CI 1.08-10.23).
Results for the polymorphic R1587K site in this gene using non parametric ã correlation analysis are shown in Table 6. For probands with CAD, this polymorphism did not influence the main CAD clinical presentations (such as MI and the severity of angina) or the degree of coronary artery atherosclerotic lesions (vessel index, stenosis index or coronary artery occlusions). There were no apparent effects of this polymorphism on the plasma lipid spectrum. However, among the sibling group, there were very significant correlations between the R1587K polymorphism and CAD, MI and angina. Thus, the increased risk of CAD and its main manifestations is associated with RR genotypes. The RR value for developing CAD among subjects with RR genotype was 4.88 (95% CI 4.14-7.62), for MI it was 6.18 (95% CI 2.26-10.1) and for angina, 3.69 (95% CI 2.00-5.18) (Table 9).
Table 7. The R219K ABCA1 gene polymorphism and artery measurements by coronary angiography. Vessel index is the number of vessels with stenosis of more than 50%: 1 = one vessel; 2 = two vessels; 3 = three vessels.

Table 8.
Genotype distribution at the R219K polymorphism of the ABCA1 gen in the sibling group either with or without coronary artery disease, angina and arterial hypertension.
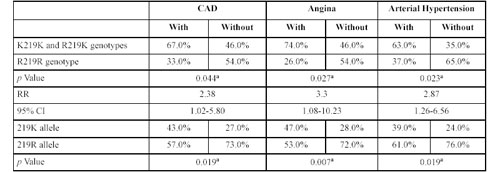
Table 9.
Genotype distribution at the R1587K polymorphism of the ABCA1 gene in the sibling group either with or without coronary artery disease, myocardium infarction and angina.
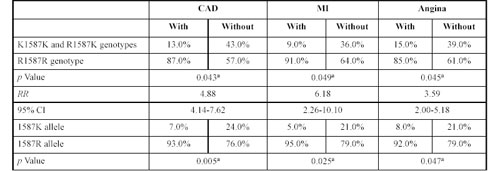
Table 10.
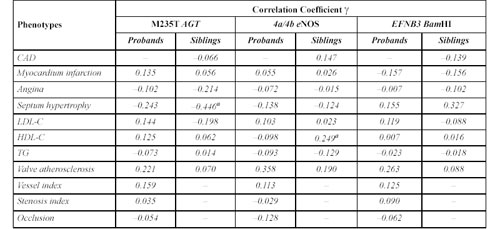
Correlation between clinical and biochemical data and the AGT, eNOS, EFNB3 genes in coronary artery disease patients and their siblings by non parametric ã correlation analyses.
Thus, among this Russian population, the ABCA1 gene R1587K polymorphism was associated with a clear risk of developing CAD for individuals with a family history of CAD. Evidently, the rare allele K is protective and reduces the risk of CAD.
Analysis of eNOS4a/4b, the M235T Polymorphism of AGT and the C/A (BamHI) Polymorphism of EFNB3. Table 10 shows the ã correlation test for the AGT polymorphism of M235T, and for eNOS minisatellite 4a/4b and EFNB3 BamHI polymorphism. For each polymorphism, there appeared to be at least one significant correlation with a given disease characteristic. However, further analyses by ÷2 and Newman-Keuls tests did not reveal any significant correlations of clinical parameters with any of the corresponding genotypes.

