


SIMULTANEOUS DETECTION OF FACTOR V LEIDEN
AND FACTOR II G20210A VARIANTS BY A
MULTIPLEX PCR ON WHOLE BLOOD
Djordjevic V, Rakicevic L, Gagic M, Nikolic A, Savic A
*Corresponding Author: Valentina Djordjevic, Laboratory for Molecular Biology, Institute of Molecular Genetics and Genetic Engineering, Vojvode Stepe 444a, P.O. Box 446, 11001 Belgrade, Yugoslavia; Tel: +381 11 3976658; Fax +381 11 3975 808; E-mail qwert@eunet.yu
page: 15
|
|
RESULTS AND DISCUSSION
Simultaneous detection of the factor V Leiden and factor II G20210A mutations is made possible by the fact that the temperatures of annealing for factor V and factor II primers are 57°C and 56°C, respectively. It required standardization of reaction conditions that would give similar amounts of amplified fragments and identifiable restriction fragments. In a series of experiments, amounts of blood, Taq polymerase and primers were varied in order to obtain approximately equal quantities of factor V and factor II PCR products.
Variation of blood volume (0.5-2.0 mL) showed that the optimal yield was obtained with 1 mL and that the smaller volumes were insufficient. Amplification decreased with higher volumes of blood, indicating that Taq polymerase could be inhibited by some blood components. Variation in amounts of Taq polymerase (1, 2 and 3 U) showed that simultaneous amplification of factor V and factor II required 2 U, more than the amplification of one fragment did. Although both factor V and factor II products were obtained after PCR, their quantities were not equal, as factor II was amplified preferentially. However, the ratio of the products changed with different amounts of primers. When the ratios of factor V and factor II primers were between 10:4 and 10:3, equal amounts of both products were obtained.
The MnlI enzyme digests the normal factor V allele into fragments 163, 67 and 37 bp long, while the mutated allele has one restriction site less and produces fragments 200 and 67 bp long. The HindIII enzyme digests the factor II mutated allele producing 322 and 23 bp fragments, while the normal allele remains undigested.
Characteristic fragments can be resolved and identified on the same polyacrylamide gel; those below 100 bp are allowed to run off the gel. The method was checked and confirmed on 20 patients with a known combination of factor V Leiden and factor II G20210A variants. Some examples of different combinations of factor II and factor V alleles are given in Fig. 1.
The factor V Leiden and factor II G20210A mutations are the most frequent causes of inherited thrombophilia, and combined detection of both mutations in a single test is of great practical help. Our method has the advantage of using only 1 mL of whole blood, without any additional treatment and primers that can be applied for separate detection of these mutations [1,2]. Multiplex PCR on 1 mL of whole blood followed by simultaneous digestion with the MnlI and HindIII enzymes not only enables precise and reliable diagnosis, but also saves the time and cost of analysis. Of no less importance is the fact that the two common mutations located in two different coagulation factor genes are simultaneously detected.
The HindIII enzyme has no restriction sites in the factor V fragment, but the MnlI enzyme used for the detection of the factor V Leiden mutation, also digested the factor II PCR product. However, simultaneous digestion can be performed, since the factor II fragment is only shortened by the MnlI enzyme by 73 bp. The number and sizes of fragments obtained under these conditions are summarized in Table 1.
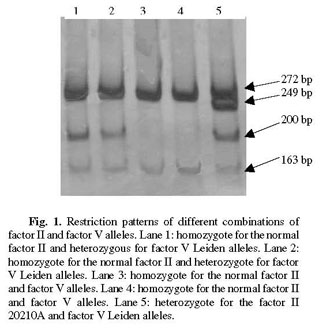
Table 1. Sizes of fragments produced by double-digestion products by the MnlI and HindIII enzymes

|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

