


GENE POLYMORPHISM OF THE DOPAMINE
RECEPTOR (DRD2) AND OF THE DOPAMINE
TRANSPORTER (DAT1) GENES IN OPIATE ADDICTION
Gareeva A*, Juriev E, Khusnutdinova E
*Corresponding Author: Dr. Anna Gareeva, Department of Genomics of the Institute of Biochemistry and Genetics, Ufa Scientific Branch of the Russian Academy of Sciences; K. Marx str. 6, 450000 Ufa, Russia; Tel: +7-3472-55-15-60; Fax: +7-3472-22-35-69; E-mail: ganna@anrb.ru
page: 61
|
|
RESULTS AND DISCUSSION
The study of associations of polymorphic loci of candidate genes with disease risk in different ethnic groups is one of the most modern approaches for determining hereditary susceptibility for psychoactive substances. With this consideration in mind, the ethnic origin of investigated individuals is of prime importance [19].
Investigations of the genetics of addiction to psychoactive substances have shown that individual peculiarities of the neuromediator system activity and its compensatory possibilities underlie formation of the present pathology under lasting influence of theses substances. An association between allelic neuromediator system gene variants and age of onset of the disease has been indicated. An association between allele A1 of the DRD2 gene and onset of alcoholism at the age of 25 years and less [6]. Because of these findings we subdivided the patient sample on the basis of ethnic affiliation, age of onset of the disease [16 years old and younger being designated as early onset, and more than 16 years old being designated as late onset, and disease duration (4 years, and less and more than 4 years)].
An Analysis of the NcoI Polymorphism of the DRD2 Gene. Results of the analysis of the NcoI polymorphism of the DRD2 gene in patients with opiate addiction and in controls are shown in Table 1. In the global patient group, three genotypes were studied. The most frequent were heterozygotes N1/N2 (0.59) and homozygotes N1/N1 (0.39).
Analysis of the results without consideration of ethnic affiliation showed differences between patient and control groups by genotypes and gene frequencies of alleles of NcoI DRD2. This showed that the relative risk of narcotization decreases in individuals with the N2/N2 genotype (p = 0.0002; OR = 0.35). For that reason the N2/N2 genotype may serve as a marker of resistance to opiate addiction. The results also showed that relative risk increases in individuals with the N1/N1 genotype (p = 0.013; OR = 1.41), so that this genotype may be regarded as an opiate addiction risk marker.
The results on ethnic affiliation of the patients showed that the N1 allele in Russians associates with early onset (p = 0.027; OR = 1.87). An association of the N1 allele with opiate addiction in Tatars was also revealed (p = 0.004; OR = 1.83), with opiate addiction onset at the age of more than 16 years old (p = 0.003; OR = 1.55) and with a narcotization duration of 4 years and less (p = 0.004; OR = 2.01). In contrast to these observations, the N2/N2 genotype is a marker of resistance to opiate addiction (p = 0.0002; OR = 0.16) with late onset in Tatars.
The literature devoted to the gene DRD2 NcoI polymorphism is contradictory. No association was determined between this gene and alcoholic delirium in Russians and Tatars from Bashkortostan [20]. However, similar research in Japanese [21] and in some aboriginal ethnic groups in Taiwan [5] showed an association of the NcoI polymorphism of the DRD2 gene with alcohol addiction.
Table 1. Genotype frequency distribution of the NcoI polymorphism of the DRD2 gene in patients and in controls
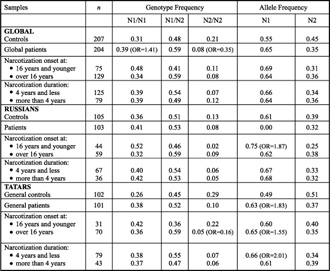
Table 2. Genotype frequency distribution of the TaqI A polymorphism of the DRD2 gene in patients and in controls.
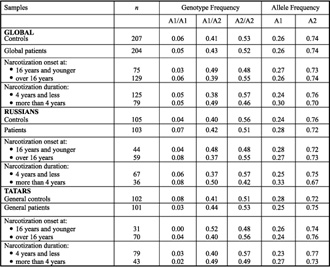
Table 3. Genotype frequency of the dopamine transporter gene DAT1 in patients and in controls
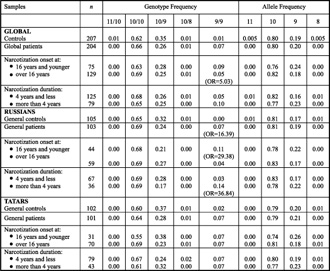
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

