


THE SULT1A1 ALLELE WITH LOW POTENTIAL FOR ESTROGEN INACTIVATION IS ASSOCIATED WITH REDUCED COLORECTAL CANCER RISK IN POSTMENOPAUSAL WOMEN
Sterjev Z1*, Josifovski T2*, Panovski M2, Suturkova L1, Dimovski AJ1
*Corresponding Author: Professor Aleksandar J. Dimovski, M.D., Ph.D., Pharmacogenetic Laboratory, Institute for Pharmaceutical Chemistry, Faculty of Pharmacy, Vodnjanska 17, 1000 Skopje, Republic of Macedonia; Tel.: +389-2-3217-580; Fax: +389-2-3290-830; E-mail: adimovski@baba.ff.ukim.edu.mk
* Contributed equally to this paper and should both be considered as first authors.
page: 43
|
|
MATERIALS AND METHODS
One hundred sporadic colorectal cancer patients, 100 newborns and 100 adults without a history of malignant disease, were investigated to determine the allele frequencies and genotype distribution of the SULT1A1 Arg213His variant in the population of the Republic of Macedonia (Table 1).
Genomic DNA was extracted from whole blood, paraffin embedded tissues and/or fresh tumors, using a QIAGEN DNA extraction kit and the procedure recommended by the manufacturer (QIAGEN AS, Oslo, Norway). The presence of the variant SULT1A1 gene was determined with a polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) procedure as follows: a fragment of 282 bp including exon 7 of the SULT1A1 gene was amplified by PCR using primers 5’-GGT TGA GGA GTT GGC TCT GC-3’ and 5’-ATG AAC TCC TGG GGG ACG GT-3’ on a GeneAmp PCR System 2700 (Applied BioSystems, Foster City, CA, USA). The PCR conditions included initial denaturation at 95°C for 10 min., 33 cycles of denaturation (95°C for 30 seconds), annealing (62°C for 30 seconds) and extension (72°C for 1 min.), and a 10 min. final extension at 72°C. The PCR products were incubated with HaeII at 37°C overnight. The resulting fragments were resolved by electrophoresis on 2% agarose gels in TBE buffer (0.89 M Ttris-HCl, 0.89 M Boric acid and 0.02 M EDTA-Na2-salt), visualized by ethidium bromide staining and recorded on digital camera. Representative results showing the detection of various genotypes in four samples are shown in Figure 1.
Comparison of allele frequencies and genotype distributions between patients and controls was done by Chi-square, Fisher’s exact test and Multivariate statistical analysis with Bonferoni correction. The genotype frequencies in patients and controls were tested if they follow the Hardy-Weinberg equilibrium.
Table 1. Some characteristics of the patient and control groups in this study.
|
|
CRC Patients (n = 100) |
Adults (n = 100) |
Newborns (n = 100) |
|
Gender:
Males
Females |
56
46 |
32
68 |
39
61 |
|
Nationality:a
Macedonian
Otherb |
87
13 |
85
15 |
59
31 |
|
Age:
<60 years
>60 years |
40
60 |
8
92 |
|
|
Family History of CRC:
Positive
Negative |
16
84 |
|
|
|
Localization:a
Rightc
Leftd |
26
60 |
|
|
|
Dukes’ Stage:a
A
B
C
D |
4
37
31
4 |
|
|
a Where data are available.
b Albanians, Turks, Gypsies, Serbs, etc.
c Caecum, ascending colon , transverse colon.
d Descending colon, sigmoid, rectum.
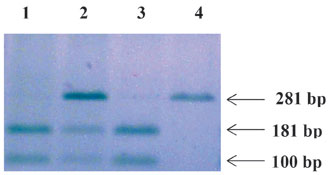
Figure 1. Agarose-gel electrophoresis showing representative results of the PCR-RFLP assay for the detection SULT1A1 Arg213His. Lanes 1 and 3: His®His; lane 2: Arg®His; lane 4: Arg®Arg.
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

