


IN SITU HYBRIDIZATION, WITH OR WITHOUT
TYRAMIDE SIGNAL AMPLIFICATION, IN
EVALUATION OF HUMAN PAPILLOMAVIRUS
STATUS INEARLY STAGE CERVICAL CARCINOMA
Kubelka-Sabit KB*, Prodanova ILj, Zografski GD, Basheska NT
*Corresponding Author: Katerina Kubelka-Sabit, M.D., M.Sci., Department of Histopathology
and Clinical Cytology, Institute of Radiotherapy and Oncology, Medical Faculty, Vodnjanska 17,
1000 Skopje, Republic of Macedonia; Tel.: +389-2-3147-902; Fax: +389-2-3111-430 ; E-mail:catkubelka@yahoo.co.uk
page: 41
|
|
RESULTS
Using CISH, HPV DNA was detected in 26 cases (33.8%). However, using ISH TSA, 20 more HPV-positive cases were detected (Table 1, Figure 2). In one case, the HPV was detected with CISH, but the result could not be confirmed with ISH TSA. Human papillomavirus types 6/11 was detected in one case (3.9%) of verrucous invasive squamous cell carcinoma by both methods. Human papillomavirus types 16/18 was detected in 20 cases (76.9%) using CISH, whereas 13 new positive cases were revealed using ISH TSA. One case was positive for HPV 16/18 using CISH, but the presence of HPV was not confirmed using ISH TSA. Human papillomavirus types 31/33/51 was detected in five cases (19.2%) using CISH. The ISH TSA system detected five new cases of HPV types 31/33 and also con- firmed the presence of HPV types 31/33 in three of the five initially positive cases. The remaining two cases were confirmed as positive only when they were retested with the probe for HPV types 31/33/51 (Table 1). Introduction of the ISH TSA system enabled detection of multiple HPV infections in four cases (Figure 3), of whom two were positive for HPV types 16/18 and 31/33/ 51 and negative for HPV types 31/33, suggesting that probably one of the HPV types present was 51. In the other two cases,multiple HPV infections were detected using probes for HPV types 16/18 and 31/33 which had not been detected by CISH (Table 1). The number of cases with a dot hybridization signal (one or a few copies of possibly integrated HPV DNA) was higher with ISH TSA than with CISH (31/45 or 68.9% vs. 12/26 or 46.2%). In five cases in which a diffuse hybridization signal was visualized with CISH (Figure 3c), more cells with a dot hybridization signal were visualized (mixed hybridization signal, Figure 3d) with the ISH TSA system (Table 1). More than five positive cells per tissue sample were detected in 37/45 cases (82.2%, Figure 2c, Figure 3d and 3f) by ISH TSA, in comparison to 19/26 (73.1%) detected by CISH (Table 1). Furthermore, in five of the seven cases in which 1-5 positive cells per tissue sample were detected by CISH, more than five positive cells were detected by ISH TSA. In four of these cases, the number of positive cells increased to more than 10. However, in three other cases, the number of infected cells was higher with CISH than with ISH TSA. When the results on HPV status obtained by the two methods were compared using Mc Nemarís test for correlated proportions, the differences for each of the parameters were statistically significant, in favor of the ISH TSA system (Table 1).
Table 1. Comparison of two in situ hybridization methods in detection and typing of HPV, evaluation of the physical state of HPV DNA, and estimation of the number of positive cells.
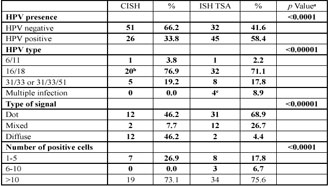

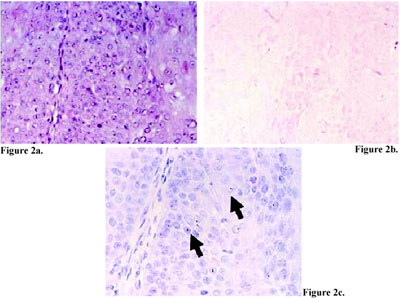
Figure 2 . Squamous cell carcinoma (a, hematoxylin and eosin stain, 400X magnification) negative for HPV DNA using CISH (b, CISH, counter stained with eosin, 400X magnification), but positive for HPV DNA type 16/18 using ISH TSA (c, ISH TSA, counter stained with hematoxylin, 400X magnification, dot hybridization signal, arrow).
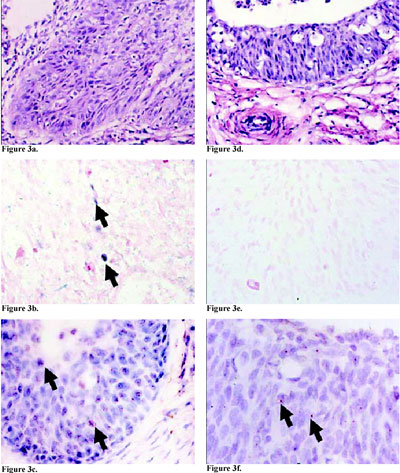
Figure 3. Squamous cell carcinoma (a and b, hema toxylin and eosin stain, 200X magnification) with double HPV infection, positive for HPV type 31/33/51 with CISH (c, CISH, counter stained with eosin, 400X magnification, arrow), and with ISH TSA (d, ISH TSA, counter stained with hematoxylin, 400X magnification), mixed hybridization signal, arrow). The tumor was negative for HPV type 16/18 using CISH (e, CISH, counter stained with eosin, 400X magnification), but positive for HPV DNA type 16/18 using ISH TSA (f, ISH TSA, counter stained with hematoxylin,400X magnification, dot hybridization signal, arrow).
|
|
|
|



 |
Number 27
VOL. 27 (2), 2024 |
Number 27
VOL. 27 (1), 2024 |
Number 26
Number 26 VOL. 26(2), 2023 All in one |
Number 26
VOL. 26(2), 2023 |
Number 26
VOL. 26, 2023 Supplement |
Number 26
VOL. 26(1), 2023 |
Number 25
VOL. 25(2), 2022 |
Number 25
VOL. 25 (1), 2022 |
Number 24
VOL. 24(2), 2021 |
Number 24
VOL. 24(1), 2021 |
Number 23
VOL. 23(2), 2020 |
Number 22
VOL. 22(2), 2019 |
Number 22
VOL. 22(1), 2019 |
Number 22
VOL. 22, 2019 Supplement |
Number 21
VOL. 21(2), 2018 |
Number 21
VOL. 21 (1), 2018 |
Number 21
VOL. 21, 2018 Supplement |
Number 20
VOL. 20 (2), 2017 |
Number 20
VOL. 20 (1), 2017 |
Number 19
VOL. 19 (2), 2016 |
Number 19
VOL. 19 (1), 2016 |
Number 18
VOL. 18 (2), 2015 |
Number 18
VOL. 18 (1), 2015 |
Number 17
VOL. 17 (2), 2014 |
Number 17
VOL. 17 (1), 2014 |
Number 16
VOL. 16 (2), 2013 |
Number 16
VOL. 16 (1), 2013 |
Number 15
VOL. 15 (2), 2012 |
Number 15
VOL. 15, 2012 Supplement |
Number 15
Vol. 15 (1), 2012 |
Number 14
14 - Vol. 14 (2), 2011 |
Number 14
The 9th Balkan Congress of Medical Genetics |
Number 14
14 - Vol. 14 (1), 2011 |
Number 13
Vol. 13 (2), 2010 |
Number 13
Vol.13 (1), 2010 |
Number 12
Vol.12 (2), 2009 |
Number 12
Vol.12 (1), 2009 |
Number 11
Vol.11 (2),2008 |
Number 11
Vol.11 (1),2008 |
Number 10
Vol.10 (2), 2007 |
Number 10
10 (1),2007 |
Number 9
1&2, 2006 |
Number 9
3&4, 2006 |
Number 8
1&2, 2005 |
Number 8
3&4, 2004 |
Number 7
1&2, 2004 |
Number 6
3&4, 2003 |
Number 6
1&2, 2003 |
Number 5
3&4, 2002 |
Number 5
1&2, 2002 |
Number 4
Vol.3 (4), 2000 |
Number 4
Vol.2 (4), 1999 |
Number 4
Vol.1 (4), 1998 |
Number 4
3&4, 2001 |
Number 4
1&2, 2001 |
Number 3
Vol.3 (3), 2000 |
Number 3
Vol.2 (3), 1999 |
Number 3
Vol.1 (3), 1998 |
Number 2
Vol.3(2), 2000 |
Number 2
Vol.1 (2), 1998 |
Number 2
Vol.2 (2), 1999 |
Number 1
Vol.3 (1), 2000 |
Number 1
Vol.2 (1), 1999 |
Number 1
Vol.1 (1), 1998 |
|
|

